*The EAU-recommended testosterone level is <20 ng/dL.3 This definition is important as better
results are repeatedly observed with lower testosterone levels compared to 50 ng/dL.4
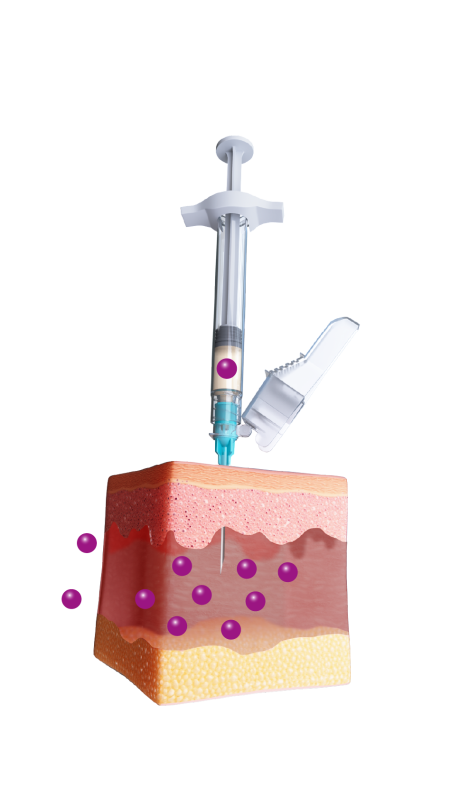
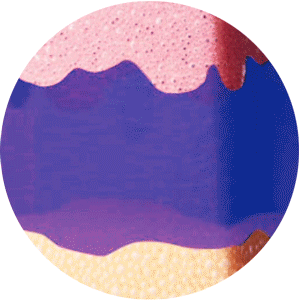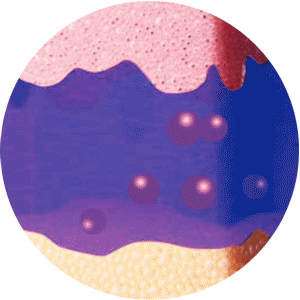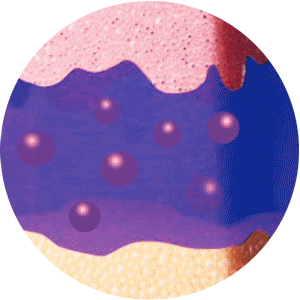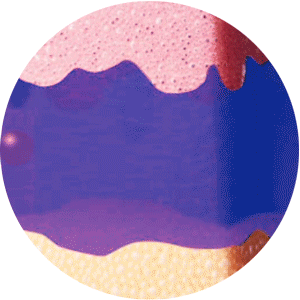
ELIGARD offers controlled and sustained release
of leuprorelin acetate between injections2

Mixture of solvent and leuprorelin molecules
contact with water molecules
in the dermis and forms a
solid in-situ depot
Continuous and extended release of leuprorelin
ELIGARD AtrigelTM provides reliability
for you and your patients:
Delayed onset
of CRPC2,4,6,7

An additional 14 days of
testosterone suppression8
Maximising drug exposure
of leuprorelin8

Subcutaneous injections lasting 1, 3, and 6 months2
*The EAU-recommended testosterone level is <20 ng/dL.3 This definition is important as better results are repeatedly
observed with lower testosterone levels compared to 50 ng/dL.4
ADT, androgen deprivation therapy; EAU, European Association of Urology; CRPC, castrate-resistant prostate cancer.
Long-term control with no breakthroughs
in over 99% of patients, reducing the potential risk of disease progression2
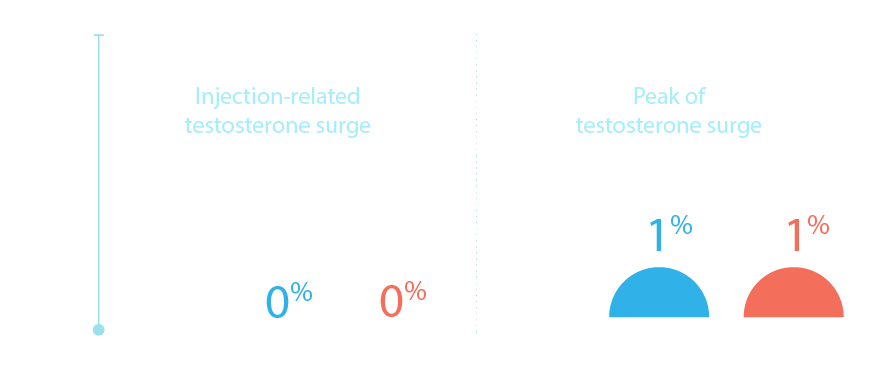

Delayed injections can lead to elevated testosterone and increased
risk of breakthroughs, which can impact progression and survival10

ELIGARD provides testosterone
suppression to <20ng/dL with
<1% of breakthroughs1,2,11–13
US, United States.
Consider the importance of treatment adherence
for your prostate cancer patients:
21-51%
mean
non-adherence
rates

More than
40%
of men
over 65
Injections may offer a distinct advantage
over oral medications in patients where
adherence is a potential concern16
*Reported mean non-adherence rates.
ADT, androgen deprivation therapy; sc, subcutaneous.
- ELIGARD SmPC. Latest version.
- Tombal B, et al. Eur Urol Suppl 2007;6:754–760.
- Cornford P, et al. EAU Guidelines. Edn. presented at EAU Paris April 2024. ISBN 978-94-92671-23-3.
- Shore ND, et al. BJU lnt. 2017;119(2):239–244.
- Crawford ED, et al. J Urol. 2021;205:554–560.
- Saltzstein D, et al. Ther Adv Urol. 2017;10(2):43–50.
- Pieczonka CM, et al. Rev Urol. 2018;20(2):63–68.
- Perez-Marrero R & Tyler RC. Expert Opin Pharmacother. 2004;5(2):447–457.
- Sartor O. Eur Urol Suppl. 2006;5:905–910.
- Sethi R & Sanfilippo N. Clin Interv Aging. 2009;4:259–267.
- Perez-Marrero R, et al. Clin Ther. 2002;24:1902–1914.
- Chu FM, et al. J Urol. 2002;168:1199–1203.
- Crawford ED, et al. J Urol. 2006;175:533–536.
- Higano CS, et al. J Urol. 2023;209(3):485–493.
- Grundmark B, et al. Eur J Clin Pharmacol. 2012;68(12):1619–1630.
- Fleshner NE, et al. Ther Adv Med Oncol. 2023;15:17588359231152845.

This website is intended for healthcare professionals only.
ELIGARD (leuprorelin acetate) is indicated for the treatment of hormone dependent
advanced prostate cancer and for the treatment of high-risk localised and locally
advanced hormone dependent prostate cancer in combination with radiotherapy.1
GL-ELIGA-0020 | July 2025 © 2025 Recordati
- ELIGARD SmPC. Latest version.
- Tombal B, et al. Eur Urol Suppl 2007;6:754–760.
- Cornford P, et al. EAU Guidelines. Edn. presented at EAU Paris April 2024. ISBN 978-94-92671-23-3.
- Shore ND, et al. BJU lnt. 2017;119(2):239–244.
- Crawford ED, et al. J Urol. 2021;205:554–560.
- Saltzstein D, et al. Ther Adv Urol. 2017;10(2):43–50.
- Pieczonka CM, et al. Rev Urol. 2018;20(2):63–68.
- Perez-Marrero R & Tyler RC. Expert Opin Pharmacother. 2004;5(2):447–457.
- Sartor O. Eur Urol Suppl. 2006;5:905–910.
- Sethi R & Sanfilippo N. Clin Interv Aging. 2009;4:259–267.
- Perez-Marrero R, et al. Clin Ther. 2002;24:1902–1914.
- Chu FM, et al. J Urol. 2002;168:1199–1203.
- Crawford ED, et al. J Urol. 2006;175:533–536.
- Higano CS, et al. J Urol. 2023;209(3):485–493.
- Grundmark B, et al. Eur J Clin Pharmacol. 2012;68(12):1619–1630.
- Fleshner NE, et al. Ther Adv Med Oncol. 2023;15:17588359231152845.

This website is intended for healthcare professionals only.
ELIGARD (leuprorelin acetate) is indicated for the treatment of hormone dependent
advanced prostate cancer and for the treatment of high-risk localised and locally
advanced hormone dependent prostate cancer in combination with radiotherapy.1
GL-ELIGA-0020 | July 2025 © 2025 Recordati