Tailored to your schedule
and your patients’ needs1,3
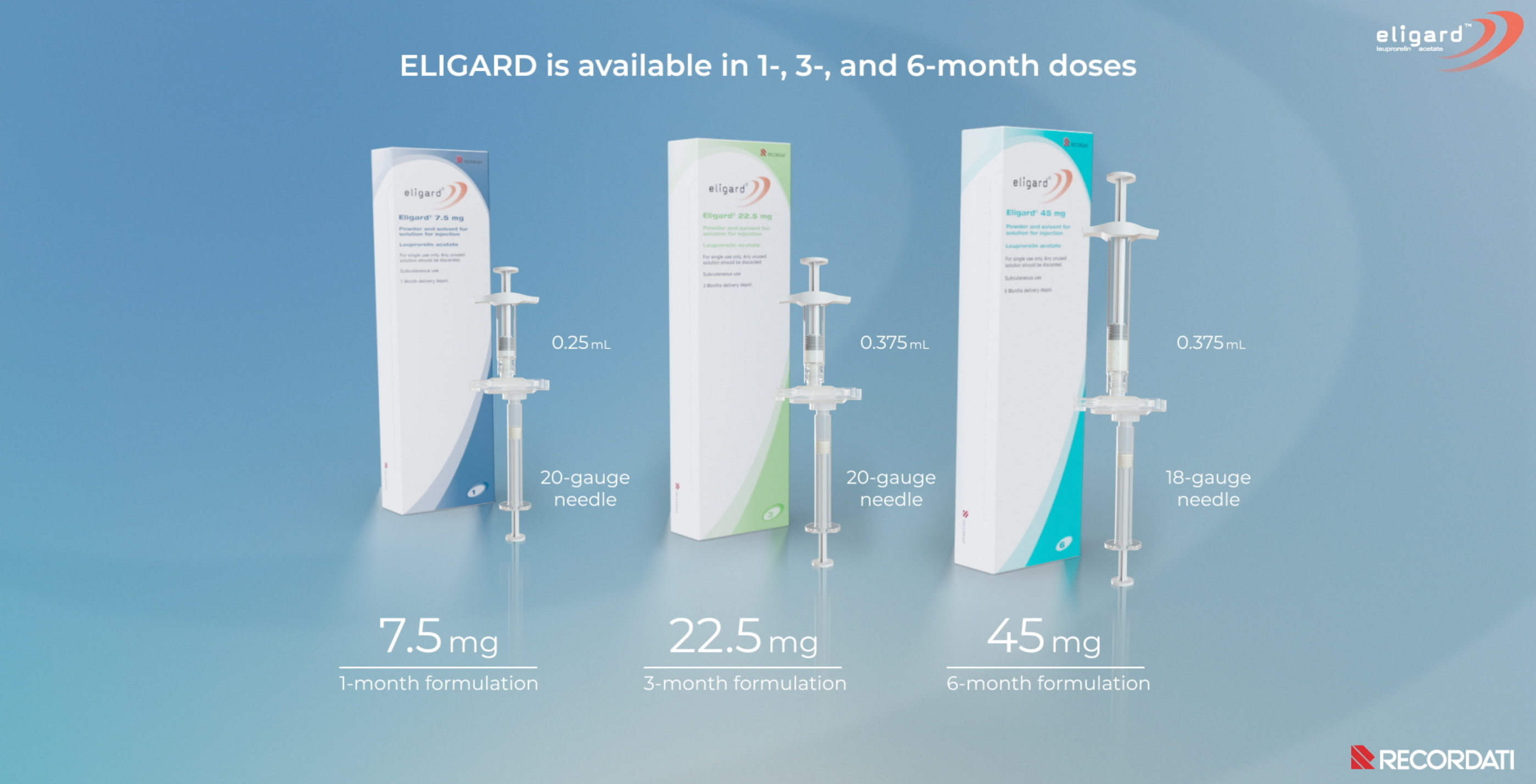
ELIGARD is available in 1, 3 and 6-month doses for subcutaneous injection and a fine needle with low injection volume1,4
7.5 mg

1-month
formulation
22.5 mg
3-month
formulation
45 mg

6-month
formulation
Longer-lasting formulations provide
flexibility for patients, with up to 6 months of treatment-free management3
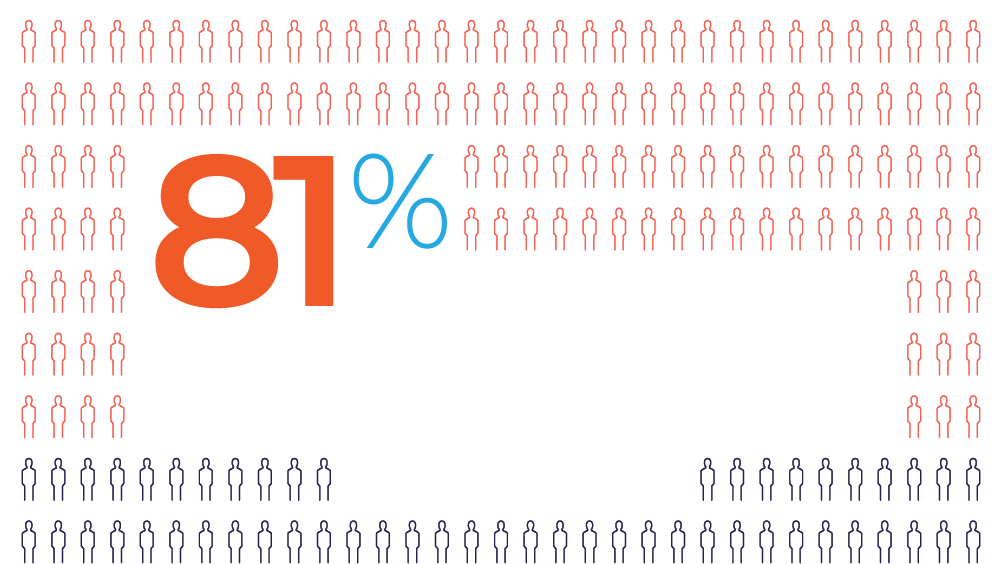
Offering multiple injection sites gives
patients greater flexibility vs a single site*1
Examples of flexible administration options1

Upper Arm

Abdomen

Upper Buttock

Thigh
*Injection sites comprise locations with adequate amounts of subcutaneous tissue that
do not have excessive pigment, nodules, lesions, or hair and have not recently been used.1
Convenient storage and handling

READY-TO-GO
You can keep ELIGARD for up to
4 weeks at room temperature
(<25°C), so it is ready for use
whenever you need it1
LONGER TERM STORAGE
Store ELIGARD in the refrigerator
(2–8°C) in its original packaging.
Acclimatise at room temperature
for approximately 30 minutes
before administration1
Pre-Connected Syringe System1
With the ELIGARD Pre-Connected Syringe
System, there’s no need to connect the syringes.
Simply
click, mix & inject1
1. CLICK

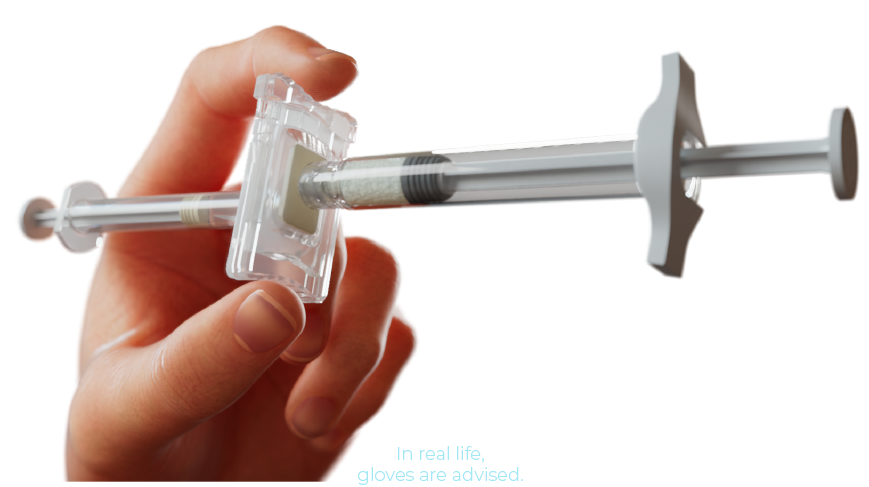
Grasp the connector button with your fingers and press until you hear a click. The two syringes are now perfectly aligned with each other.
2. MIX

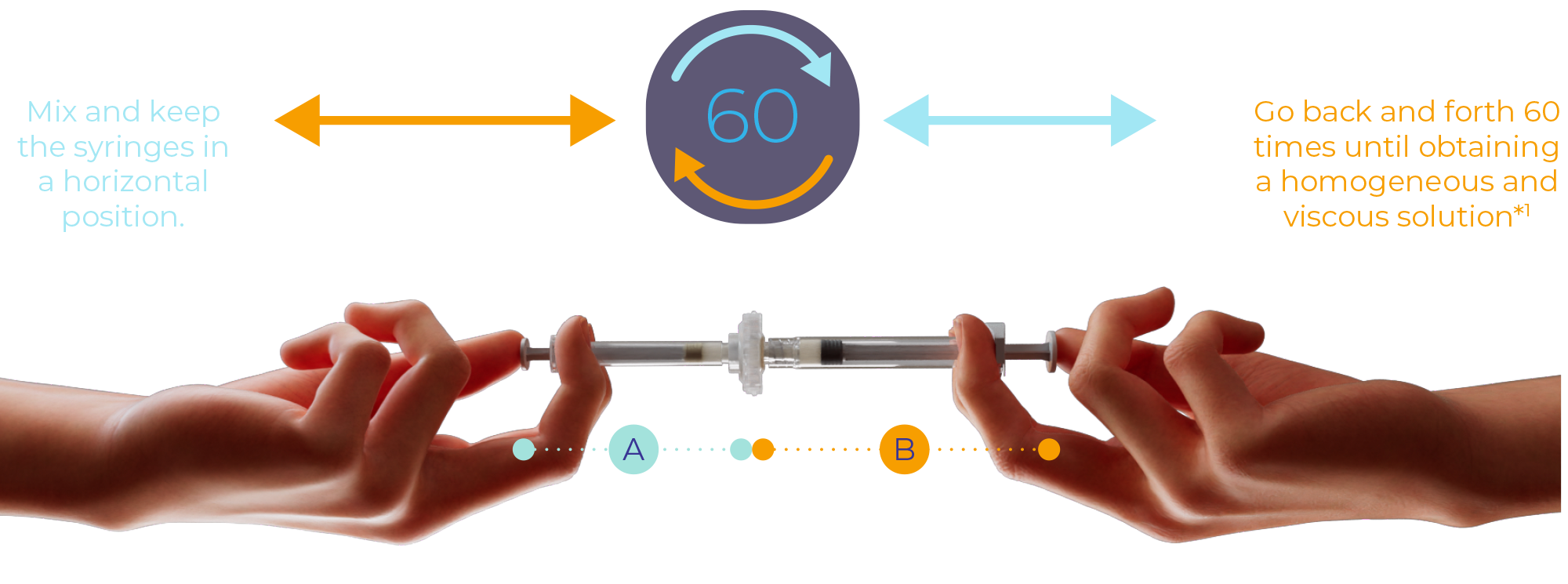
Without bending the syringe system, gently and slowly go back and forth for 60 cycles until obtaining a homogeneous and viscous solution.†
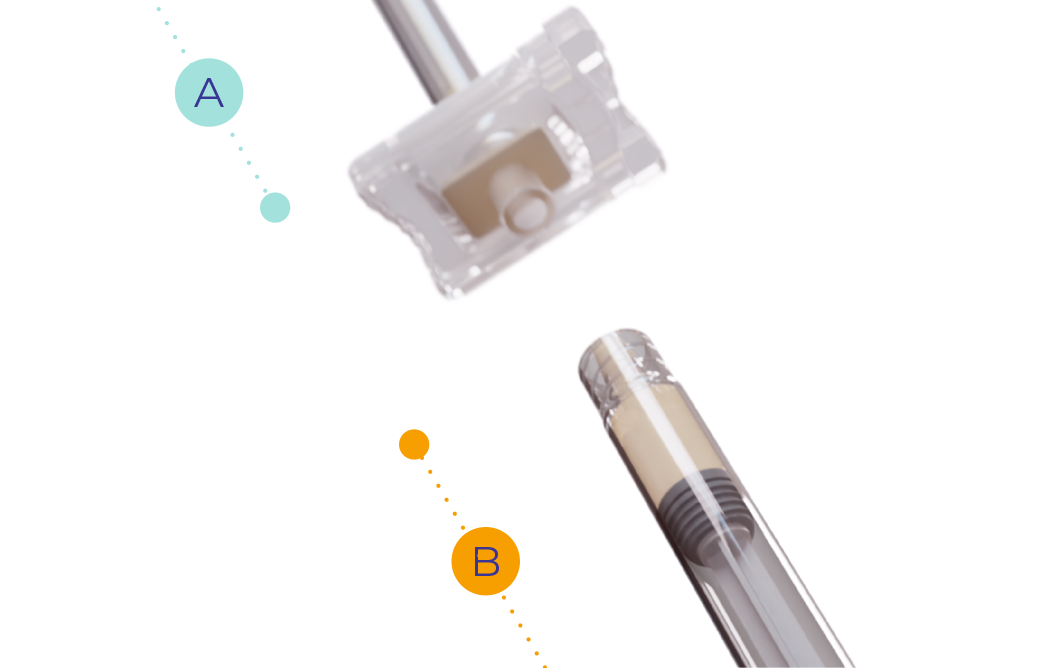
Slightly lower the plunger of syringe B to prevent product from leaking out.
syringe B.
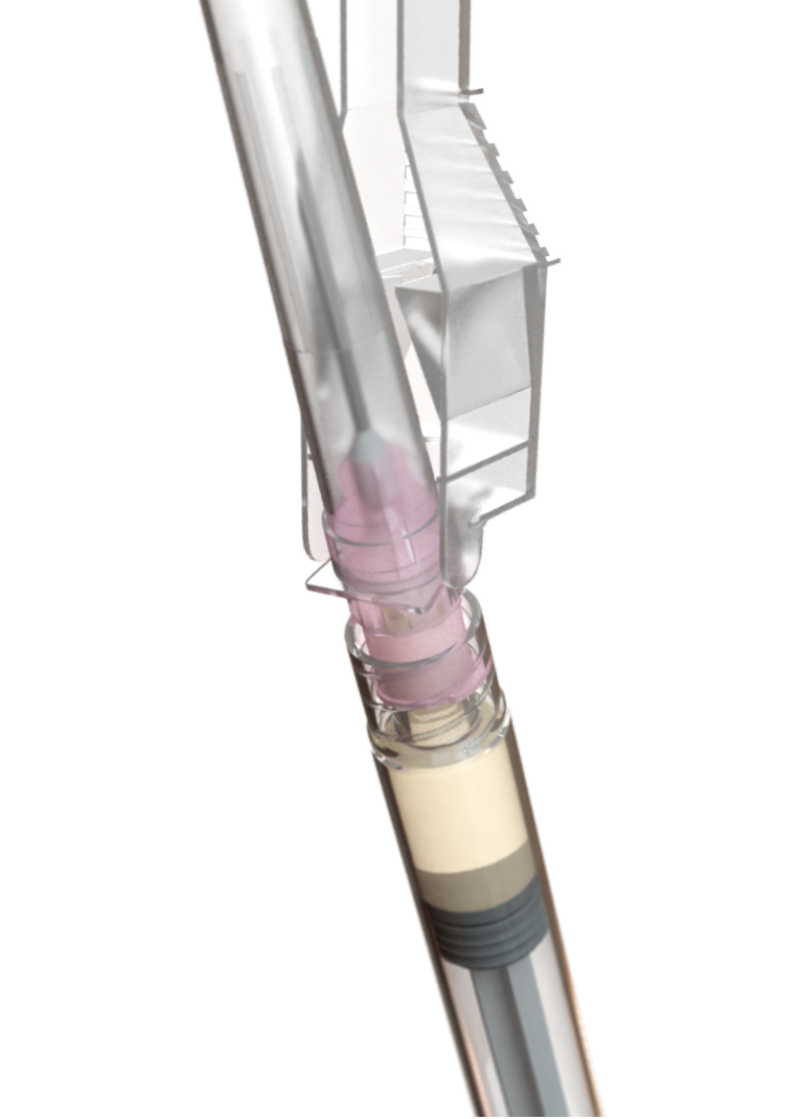
Secure the needle with safety shield onto syringe B by turning it clockwise, without overly tightening.
Prior to administration, purge any large air bubbles from Syringe B.

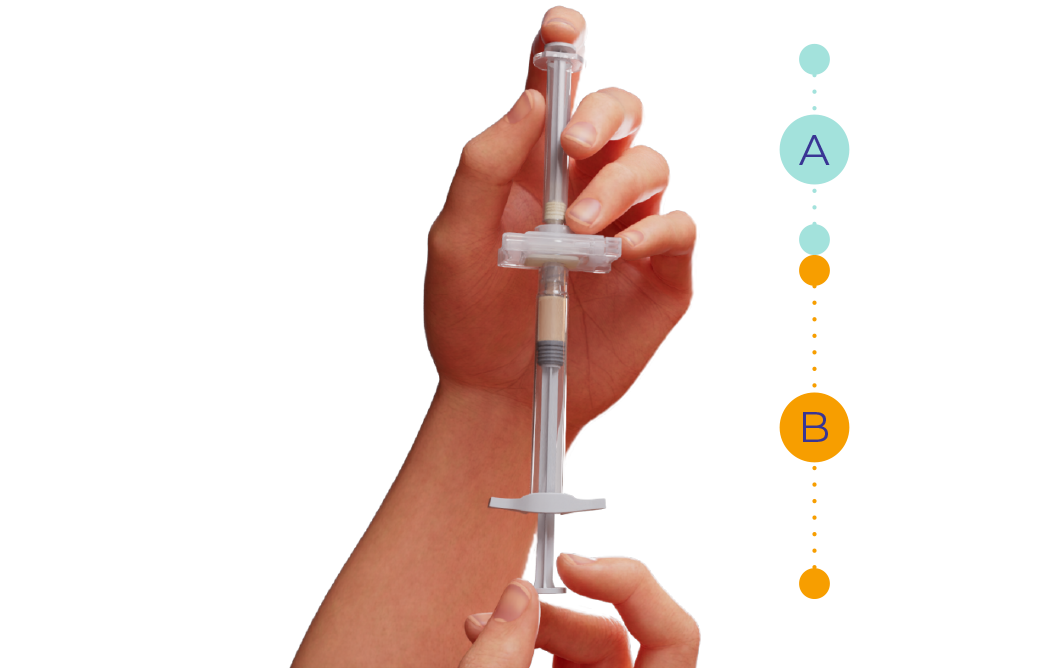
Hold the syringes in a horizontal position and slowly and gently transfer the liquid from syringe A to the powder of syringe B.
Without bending the syringe system, gently and slowly go back and forth for 60 cycles until obtaining a homogeneous and viscous solution.†

Slightly lower the plunger of syringe B to prevent product from leaking out.


3. INJECT

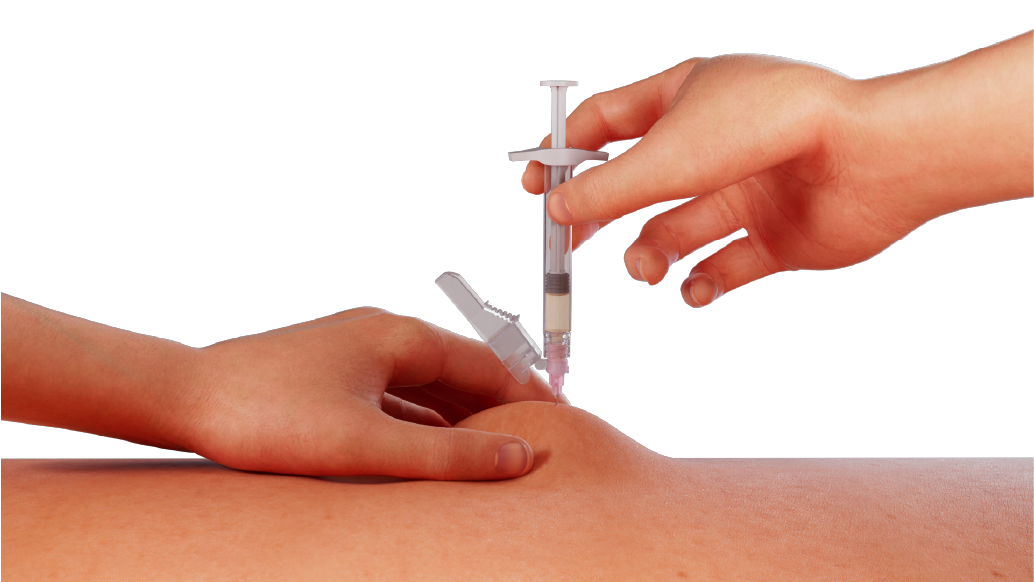
Select an appropriate injection site and cleanse it with an alcohol swab.
Administer ELIGARD immediately by grabbing the skin around the injection site, inserting the needle at a 90° angle, and slowly injecting the syringe contents until syringe is completely empty.1
Watch the full ELIGARD
administration video here


94%
of healthcare
professionals would
highly recommend
Eligard EPSS system6
*For Adverse Event reporting and full Product Information including administration instructions, please visit
[URL link to local prescribing information]
†A single cycle represents 1 push of syringe A and 1 push of syringe B. Sixty cycles are performed during mixing
References
- ELIGARD SmPC. Latest version.
- Sartor O. Eur Urol Suppl. 2006;5:905–910.
- Ouzaid I, et al. Progrès en Urologie. 2011;21:866–87
- Center for Drug Evaluation and Research. ELIGARD 45 mg Injection Suspension Approval Package. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021731s000_Eligard_AdminCorres.pdf. (Last Accessed: July 2025).
- Schulman C. BJU Int. 2007;100 (Suppl. 1):6–11
- Fortgeschrittenes Prostatakarzinom: Neues Spritzensystem vereinfacht die hoch wirksame ADT mit Leuprorelin-Gel-Implantat.

This website is intended for healthcare professionals only.
ELIGARD (leuprorelin acetate) is indicated for the treatment of hormone dependent
advanced prostate cancer and for the treatment of high-risk localised and locally
advanced hormone dependent prostate cancer in combination with radiotherapy.1
GL-ELIGA-0020 | July 2025 © 2025 Recordati





